Our funded research
The COVID-19 Immunity Task Force (CITF) was proud to fund Canada’s leading minds in the areas of immune science, seroprevalence studies, optimization of immunologic testing, vaccine surveillance, pediatric vaccination, booster vaccinations, various cross-cutting themes, and immunity modelling. The CITF sponsored 120 core studies, although several of those studies were granted extensions to evolve to respond to new pandemic conditions and as such had multiple foci. In collaboration with multiple partners, all of the studies funded by the CITF fed the scientific body of knowledge about COVID-19 and contributed important data and information to support a comprehensive and coordinated response to the COVID-19 pandemic.
CITF-funded research also supported the scientific training and professional development of the next generation of researchers. CITF funding resulted in over 63 postdoctoral fellows, 52 PhD students, 49 master’s students, and 111 undergraduate students being involved in pandemic scientific research.
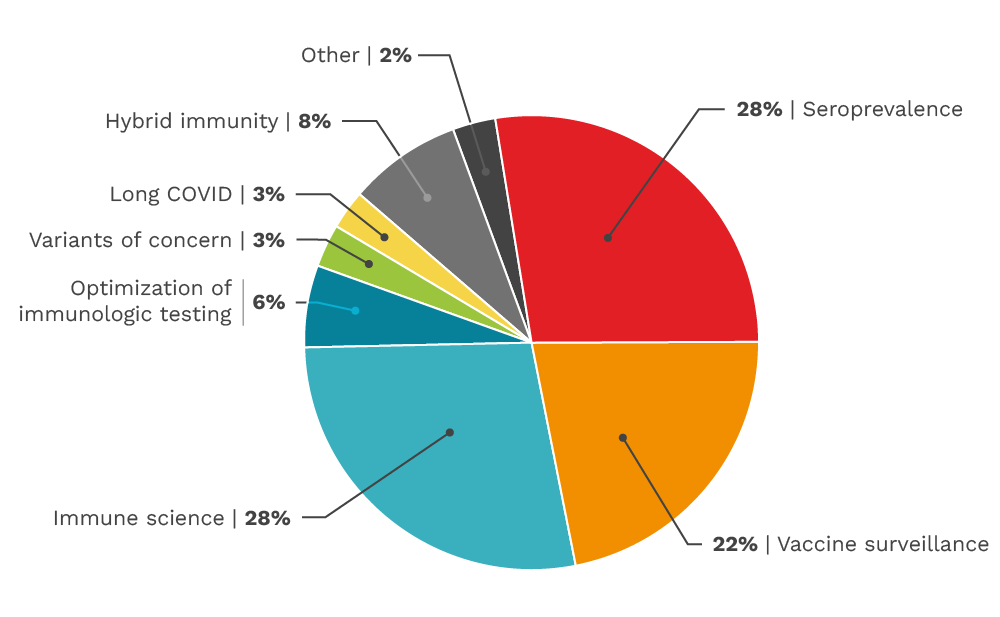
Our funded research by category
The CITF funded diverse categories of SARS-CoV-2 research across Canada from basic immune science to vaccine surveillance. This pie chart gives a basic overview of the number of studies we funded per category, although several studies straddle multiple categories.
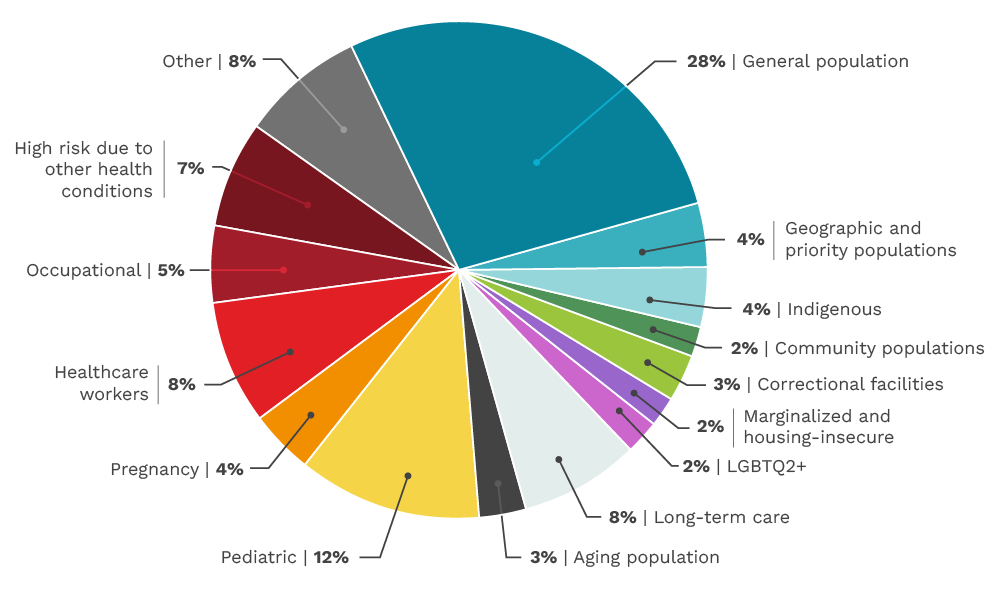
Our funded research by population
CITF-funded studies included a wide variety of different populations across Canada from pediatric to long-term care. The CITF made efforts throughout its mandate to address Equity, Diversity, and Inclusion (EDI) within the study portfolio. Over the four years, the CITF supported studies in many different equity deserving populations including children and older Canadians, Indigenous communities, those living in correctional facilities and in long-term care homes, those experiencing housing insecurity, those working high risk occupations (e.g. front-line food workers), those from 2SLGBTQ2+ communities, and those at higher risk due to other health conditions. The total number of studies in these populations constituted over 50% of the CITF study portfolio. This pie chart gives a basic overview of the number of studies we funded by population.
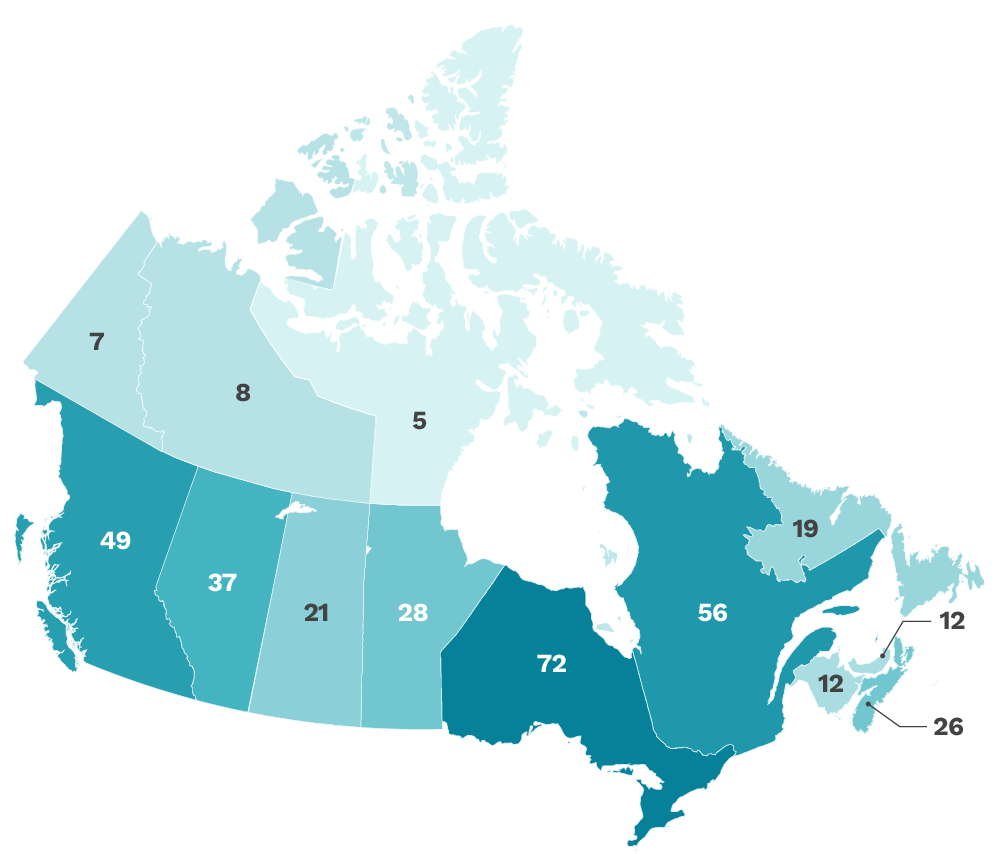
Our funded research covers all of Canada
The CITF aimed to be as inclusive of all Canadians as possible, and including with respect to geography. We funded studies from coast-to-coast-to-coast across Canada. This map shows the number of research projects that were active in each province and territory.

An important part of our Task Force’s mandate was to share information with other researchers, the public and policy makers. We used Maelstrom, a research group based at the Research Institute of the McGill University Health Centre in Montreal that developed a standard approach to documenting and disseminating epidemiological study metadata. The links to Maelstrom throughout our funded research pages may be of interest to researchers, as for each CITF-funded study it lists: general study characteristics, targeted populations, data collection events, and data dictionaries.
We supported research in priority areas
Seroprevalence
We supported seroprevalence studies focused on assessing how many Canadians had antibodies to SARS-CoV-2, the virus that causes COVID-19. People with antibodies either likely a) had SARS-CoV-2 and developed some form of immunity (see our immune science area), what is called “infection-acquired immunity,” b) were vaccinated against COVID-19 and had what is called “vaccine-induced immunity” or c) had an infection and were vaccinated (in either order) and had what is called “hybrid immunity”. Estimating seroprevalence continued to be very important in the vaccine era. Vaccine rollout in the real world did not follow ‘textbook’ results, due to the rise of variants of concern, the deviation of dosing schedules from what was used in clinical trials, and potential mix & matching of different types of vaccines, and then later, booster vaccinations. Seroprevalence continues to be a useful broad mechanism to survey people’s immune responses at a macro, population level.
Vaccine surveillance
All vaccines approved in Canada go through rigorous testing during clinical trials and are given approval by Health Canada prior to use. That said, as millions of Canadians have been vaccinated against COVID-19, the ongoing monitoring of both the effectiveness and safety of the various vaccines authorized in this country is of utmost importance. The CITF contributed to ongoing monitoring and launched several studies relating to comprehensive vaccine surveillance efforts across Canada. A consortium of Canadian organizations, including the Public Health Agency of Canada (PHAC), the Canadian Research Immunization Network (CIRN), the National Advisory Committee on Immunization (NACI) and the CITF, collaborated through the CITF’s Vaccine Surveillance Working Party to identify studies that supported the safety and effectiveness of COVID-19 vaccines across Canada.
Immune science
Despite considerable advances in our understanding of the immune system in relation to SARS-CoV-2 and COVID-19 since the beginning of the pandemic, there remain many questions related to understanding immunity. Individuals who become infected generate antibodies to the virus, but evidence has shown that antibodies are not fully protective from future reinfection. As variants of concern with immune evasive properties emerged, antibodies generated by vaccines also proved to not be fully protective against reinfection, although they continued to offer good protection against severe disease and death. This is why bivalent vaccines were created and rolled out and why continued booster dosing will likely be required. We supported research to ascertain the complexities of the immune response, whether it prevented re-infection, and how long that immune response lasts.
Optimization of immunological testing
All the work of the above priorities, whether it was related to monitoring trends in SARS-CoV-2 infection with immune measures and/or assessing the degree and durability of immune protection from infection or vaccines, was dependent on accurate measures of immunity. Ensuring precise and trustworthy immune testing was therefore central.
Pediatric vaccination
The research we catalyzed and funded in this category included ongoing safety monitoring to ensure we knew immediately if ever an issue had arisen. Our funded research looked at a variety of questions such as: What are the effectiveness and immunogenicity of SARS-CoV-2 vaccination in children (be they healthy, immunocompromised, have cardiovascular complications, etc.)? What is the effect of SARS-CoV-2 vaccination on children who have already had Multisystem Inflammatory Syndrome in children (MIS-C) or long COVID symptoms? How often will children need boosters? What is the durability of vaccine-induced immune responses (humoral, cellular, absolute antibody level, neutralizing antibodies, cellular responses, recall responses) in children?
Boosters
With growing evidence that the immune response generated by COVID-19 vaccines was efficacious against death, hospitalization, symptomatic infection and transmission, the question arose as to the duration of this protection. The answer to this question continues to change as variants of concern evolve and spread. Monitoring and modelling the rate of decline of immune protection from vaccination (clinical outcomes, humoral immunity and cellular immunity), together with information on the changing nature of SARS-CoV-2, informed the timing and type of booster vaccines to prevent a resurgence of COVID-19. In addition, research looked at whether the need and timing of booster doses would be different in the general population and in various specific sub-groups such as long-term care residents, those with other health conditions and children.
Cross-cutting themes
This category may be a bit of a catch-all, but an important one nonetheless. With over 120 studies in the field, comparing results was very interesting. However, comparisons proved tricky when studies used different measurements. The CITF therefore worked on generating measures of immunity that could be compared between studies. The correlates of protection (measurable signs that a person is immune, in the sense of being protected against disease or death) needed to be better understood, whether with respect to cellular immunity or humoral (antibody) levels following infection and vaccination. The duration of protection after one, two or three vaccines was also a question. This information was required to determine the need for and optimal timing of further vaccination and to prevent or mitigate future waves of SARS-CoV-2 infection in a timely manner.
Immunity modelling
Close monitoring of the level of SARS-CoV-2 immunity in different settings across Canada and internationally was required to sustain workplace, school and business re-openings and avoid further outbreaks of COVID-19. The CITF requested that all its funded studies share anonymized data with its Secretariat, to integrate it into immunity monitoring and mathematical modelling work. This modelling provides information on immunity to guide decisions about public health measures. Moreover, information gathered from studies on a broad array of factors such as the duration of infection and/or vaccine-induced immunity or the impact of new variants of concern on vaccine effectiveness, was incorporated into our mathematical models that explored possible pandemic scenarios to help policy makers understand levels of effective population immunity.