About the CITF Databank
The CITF is supporting 120 studies to generate knowledge about immunity to SARS-CoV-2. The subjects addressed by these studies include the extent of SARS-CoV-2 infection in Canada, the nature of immunity, vaccine effectiveness and safety, and the need for booster shots among different communities and priority populations in Canada.
The CITF Databank was developed to further enhance the impact of our funded studies by allowing additional research using the data collected for CITF-supported studies. We centralize population-level seroprevalence estimates and harmonize individual-level data deposited in the CITF Databank to provide the research community, both in Canada and around the world, with a wide variety of standardized COVID-19 data.

Two types of data in the CITF Databank
AGGREGATE DATA
The CITF Databank includes statistical aggregates from some studies in the form of seroprevalence estimates. Some of these estimates are stratified by characteristic, such as geographical region and age group.
Access process
Visit the CITF Dataverse to download the data and see our Seroprevalance in Canada webpage to explore the plotted data.
Individual-level data
The CITF Databank centralizes and harmonizes individual-level data from CITF-funded studies that have met all ethical requirements to deposit data in the CITF Databank and have completed a data sharing agreement.
Learn about the contents and how to access it on this page.

CITF Databank | Individual-level data included
Populations in the CITF Databank
The following plot shows the distribution of primary and secondary populations of studies expected in the CITF Databank. To view the specific studies in each category, click on a secondary population in the outer ring. To return to the full starburst graphic again, double-click in the centre.
About data harmonization
To facilitate harmonization, studies were asked to use the CITF-created Core Data Elements (CDE): a set of standardized survey questions and laboratory measurements intended to capture essential information related to COVID-19 epidemiology and immunity.

The CITF CDE include:
- Socio-demographic questions: individual identity (age, sex, gender, ethnicity, education, etc.), location of residence and living conditions, general health, and occupation.
- COVID-19-related questions: participant behaviors, travel history, symptom history and infection status.
- Vaccine dosage questions: participant vaccination history.
- Serological and cell-mediated immunity assay results.
For more information about our CDE and CITF data sharing policies and procedures, visit our Tools and Information for Researchers page.
The CITF works with Maelstrom Research, a research group based at the Research Institute of the McGill University Health Centre in Montreal that has developed a standard approach to documenting and disseminating metadata from epidemiological studies. Maelstrom Research has supported the CITF in standardizing and harmonizing the data collected by CITF-supported studies.
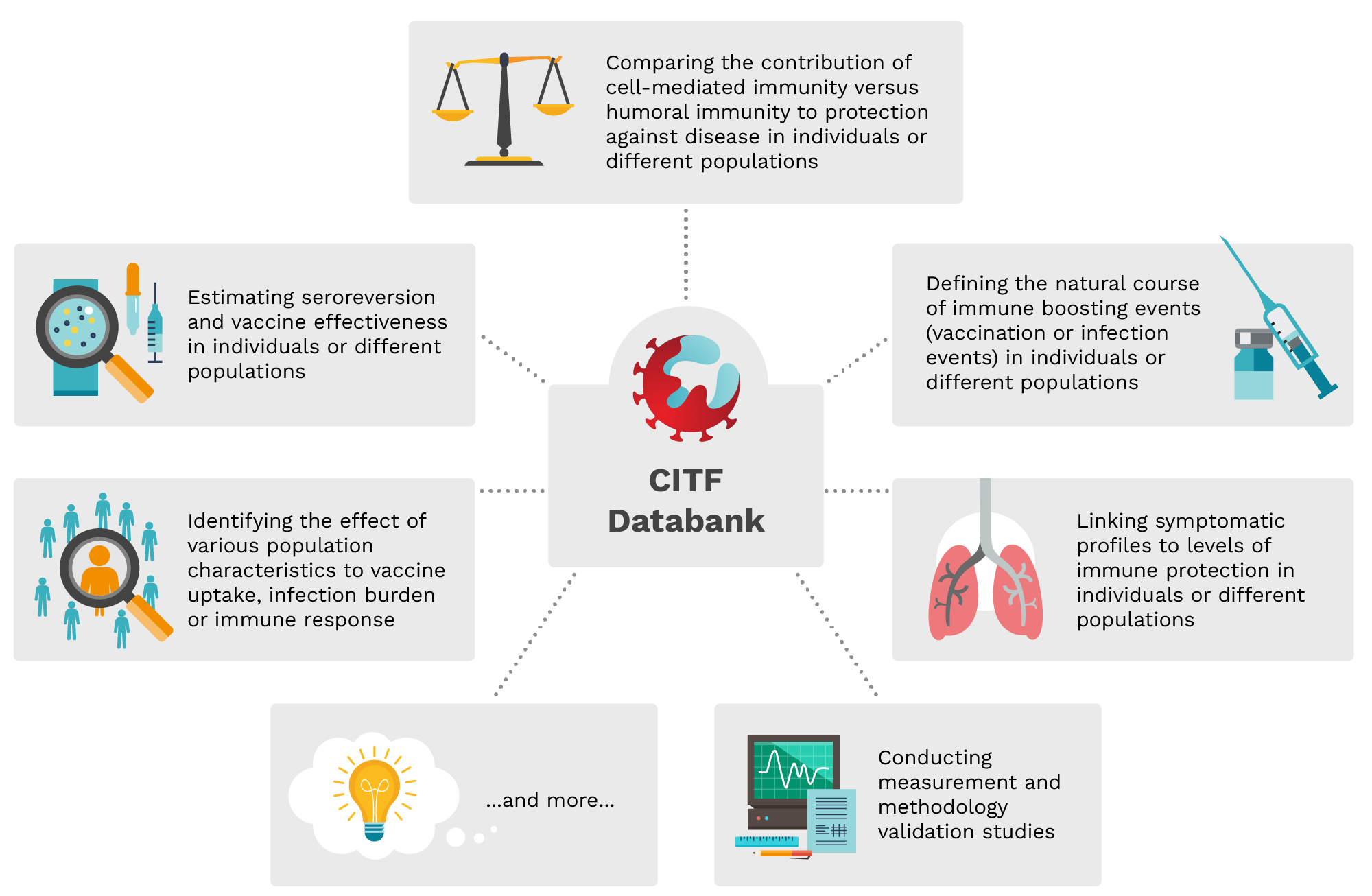
CITF Databank | Possible uses for individual data
CITF Databank | Accessing individual-level data
The CITF Databank contains de-identified individual-level data, which is available through restricted access; researchers wishing to use these data must submit a request to the CITF. To access the data in the CITF Databank, please enter the CITF Data Portal. The access process is free of charge.
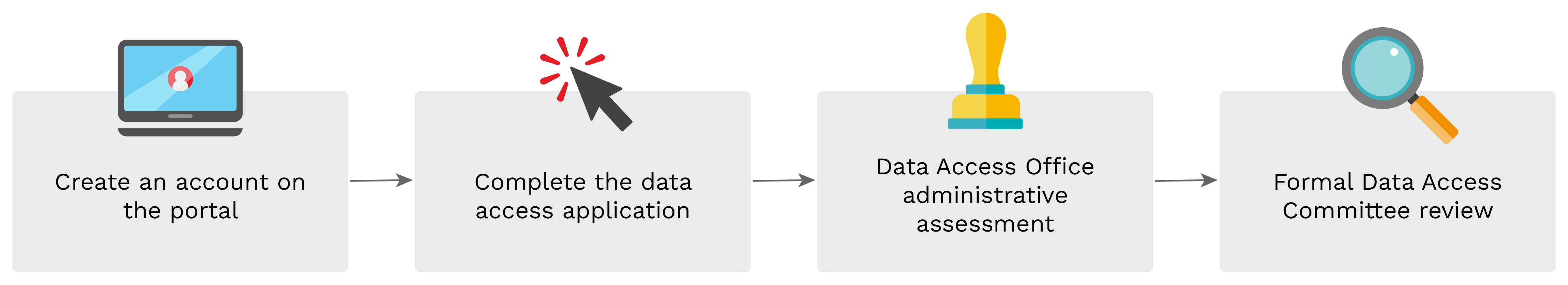
Applications for access to the CITF Databank are accepted and assessed by the CITF Data Access Office and Data Access Committee on an ongoing basis. For a more detailed description, please see the Data Access Procedure.
CITF Data Access Committee
The CITF Data Access Committee (DAC) is guided by the CITF’s Data Access Policy to assess the feasibility of the research proposal submitted in support of a data access request, and the compliance of the proposed research with CITF Guiding Principles.
DAC MEMBERS
Mr. Alexander Bernier (committee chair)
Academic Associate, Centre of Genomics and Policy
Dr. Mark Brockman
Professor, Faculty of Health Sciences, Simon Fraser University
Dr. Chelsea Gabel
Faculty of Social Sciences, McMaster University
Member, Gilbrea Centre for Studies in Aging, Faculty of Social Sciences
Director, McMaster Indigenous Research Institute, Faculty of Social Sciences
Associate Professor, Health, Aging & Society, Faculty of Social Sciences
Joint Appointment, Indigenous Studies, Faculty of Social Sciences
Canada Research Chair in Indigenous Well-Being, Community Engagement, and Innovation, McMaster University
Dr. Jeff Kwong
Senior Scientist and Program Leader, Populations and Public Health Research Program, ICES
Scientist, Public Health Ontario
Family Physician, Toronto Western Family Health Team
Professor, Department of Family and Community Medicine and Dalla Lana School of Public Health, University of Toronto
Mr. Philippe Latouche
Patient partner and Long COVID advocate, lived experience with Long COVID
CITF data governance
To facilitate transparency and accountability in the management of the CITF Databank, the CITF has developed a Data Governance Framework. This framework establishes the mechanisms for administration, custodianship and sharing of data deposited in the CITF Databank.
RESOURCES:
Data Access Policy
Data Access Agreement
Data Security Policy
Data Access Procedure
Tools and Information page