At the beginning of the pandemic, SARS-CoV-2 infections in children and adolescents in Canada were rare and generally very mild compared to adults. However, with the emergence of Omicron in December 2021 the weekly number of new COVID-19 cases in those under the age of 18 spiked to 55,956, accounting for 19% of the total number of COVID-19 cases in Canada (1). This change has been attributed to the increased transmissibility of Omicron and low vaccine uptake among children.
In this month’s research synthesis, we surveyed research findings, including those funded by the CITF, to address the following questions:
- How many children have really been infected?
- Are there adverse outcomes following COVID-19 in children and what have they been?
- What are the factors affecting COVID-19 vaccine acceptance and uptake in children? What percentage of children have been vaccinated against COVID-19?
- Have there been adverse events after vaccination in children and, if so, what have they been?
- What do the CITF-funded experts in the field of pediatrics foresee as challenges with respect to COVID-19 among children?
SARS-CoV-2 seroprevalence due to infection in children and teens across Canada
Seroprevalence increased considerably in the most recent waves of the pandemic due to the spread of Omicron and its subvariants (2). Dr. Manish Sadarangani’s CITF-funded study, Severe acute respiratory syndrome-Related coronavirus 2 prevalence in children and youNG adults in British Columbia: An observational study (SPRING), found that seroprevalence due to infection in children under the age of 9 was 7.8% between September and November 2021 – pre-Omicron – and rose to 42.3% between March and May 2022 (3). By the summer, between June and August 2022, the same SPRING Study found that over half of participants had infection-acquired antibodies, ranging from 46.4% (ages 5-9) to 57.7% (ages 10-14) (4). Based on serosurveys conducted by the British Columbia Centre for Disease Control (BCCDC) between July and August 2022, infection-acquired seropositivity among children and teens under 17 in that province was greater than 60% (5).
Estimates from Montreal echo those in B.C.. Dr. Kate Zinszer’s CITF-funded Children and COVID-19 Seroprevalence (EnCORE) study found that infection-acquired seroprevalence in the pre-Omicron era, between November and December 2021, was 10.6% for children below the age of 17. More specifically, it was 6.8% for children ages 2-4; 13.2% in children ages 5-11; and 8.7% in adolescents ages 12-17 (6). However, between May and September 2022, once Omicron hit (7), the average seroprevalence rose to 58.0%.
The EnCORE study also looked at the likelihood of seroconversion during Omicron – meaning the likelihood that a child/teen went from never having had an infection to contracting SARS-CoV-2. The team found that previously seronegative children were about 9 to 12 times more likely to seroconvert, developing infection-acquired antibodies, during the period dominated by Omicron variants (May to October 2022) than in the pre-omicron period (unpublished results).
The ENCORE study looked at the duration of infection-acquired antibodies in a child’s system. Following infection, children have antibodies against SARS-CoV-2 for a median time of 7.5 months, after which the antibodies become undetectable, indicative of waning immunity (8).
Dividing the pandemic into three distinct phases, Figure 1 (below) illustrates the exponential growth in the number of severe cases of COVID-19 among children (under 19 years old) since the start of the Omicron era.
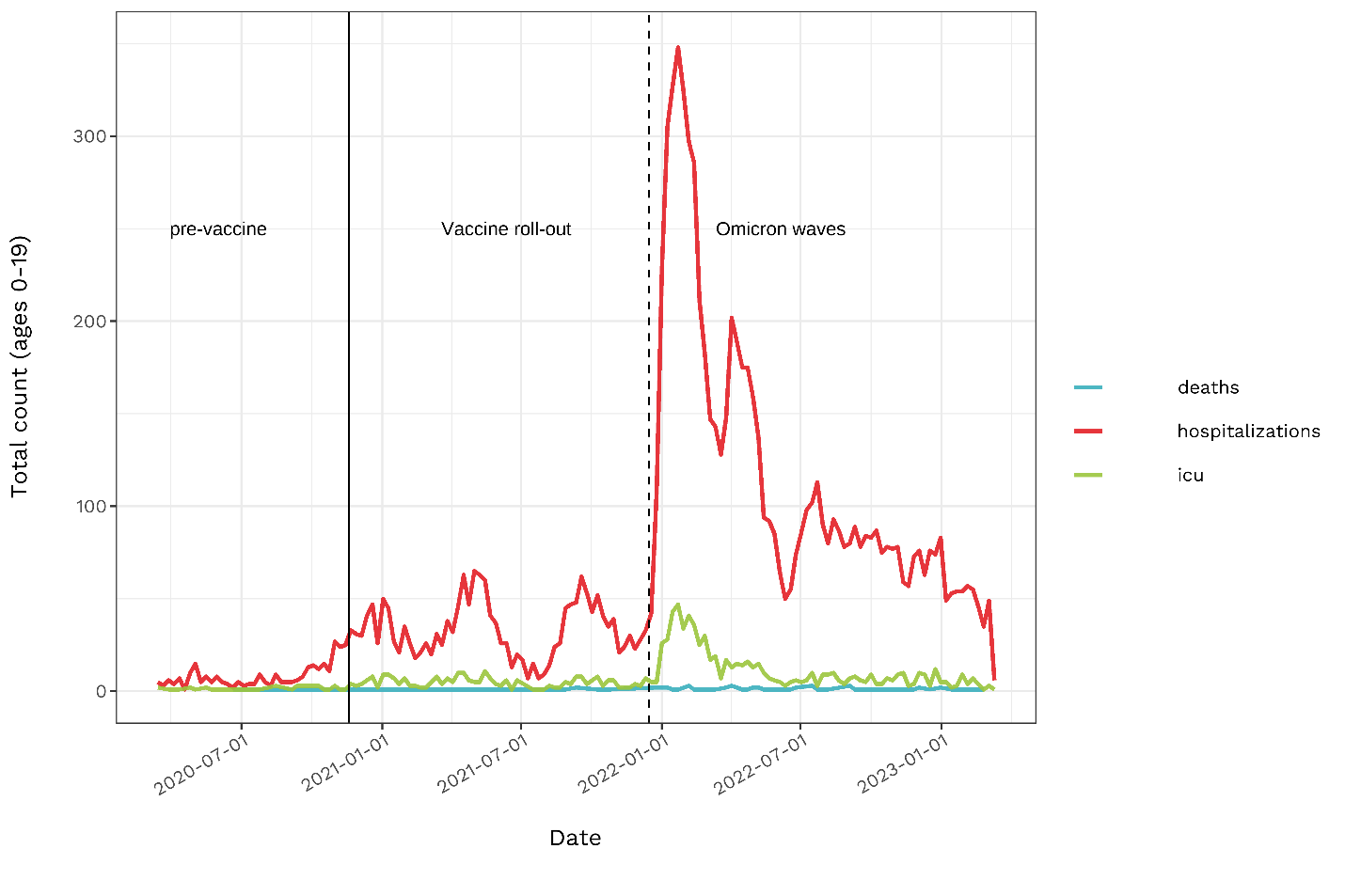
Figure 1. Total number of deaths, hospitalizations, and ICU admissions due to COVID-19 in children under 19 years of age over the course of the pandemic (1).
Adverse effects of COVID-19 on some children and teens
While it is known that adults can develop complications following severe COVID-19 disease, there is increasing evidence that children can also suffer from adverse outcomes including myocarditis (inflammation of the heart), pericarditis (inflammation of the sac that goes around the heart), multisystem inflammatory syndrome in children (MIS-C), and long COVID. Studies showed that COVID-19 vaccines decrease the risk of severe COVID-19 and complications (9-11).
Myocarditis and pericarditis
Myocarditis and pericarditis have been more common after having COVID-19 than after being vaccinated against COVID-19 (12-14). One study showed that, among males aged 5–11, the incidences of myocarditis and/or pericarditis were 12.6–17.6 cases per 100,000 after infection, but only 0–4 cases after the first mRNA vaccine dose and no cases after the second mRNA dose (12). Among males aged 12–17, incidences were much higher, after infection (with 50.1–64.9 cases per 100,000) and after the second dose (22.0–35.9 cases per 100,000) compared to the first dose (2.2–3.3 cases per 100,000) (12). Incidents in females aged 5-11 were much lower, with 5.4–10.8 cases per 100,000 after infection (12).
MIS-C
In Canada, 269 cases of MIS-C were reported to the Public Health Agency of Canada between March 11, 2020, and October 2, 2021. 53% were linked to COVID-19 (lab-confirmed cases or epidemiologically-linked with COVID-19 cases) (15). Among those children hospitalized with MIS-C, 95% had not been vaccinated (18).
In addition, having received a COVID-19 vaccine has been shown to reduce the chances of MIS-C or reduce the severity of symptoms. One study 12- to 18-year-olds demonstrated that a two-dose series of Pfizer-BioNTech vaccine was 91% effective at preventing MIS-C (10). None of the vaccinated children required respiratory or cardiovascular life support, compared with 39% of unvaccinated children with MIS-C (10).
Long COVID
Among the general population, two doses of vaccine were associated with a lower risk of long COVID compared to no vaccination or one vaccine dose (16). Compared to adults, children have a lower risk of persistent COVID-19 symptoms, but the risk is not negligible (17). Risk factors for children include severe symptoms with the initial infection, not being vaccinated, and having an unhealthy weight (body mass index in the 85th percentile or greater for age and sex) (17). As the risk of severe outcomes from SARS-CoV-2 infection far outweigh the risks of vaccination, vaccines are highly encouraged for children.
Vaccine coverage in children and teens
COVID-19 vaccine coverage in the pediatric populations varies between countries, but, in general, is substantially lower than the uptake in the general adult population. Data from the United States (18) and United Kingdom (19), alongside that from Canada, as of March 8, 2023:
| US | UK | Canada | |||||||
| Population | Age | One dose | Two doses | Booster dose | One dose | Two doses | One dose | Two doses | Booster dose* |
| Children and adolescents | <2 | 8% | 4% | 0.3% | |||||
| 2-4 | 11% | 6% | 0.4% | 9.6% | 5.3% | 5.2% | |||
| 5-11 | 40% | 33% | 4% | 11% | 7% | 52% | 40.6% | 9.5% | |
| 12-15 | 72% | 62% | 7% | 47% | 35% | 83.9% | 79.6% | 9.6% | |
| 16-17 | 62% | 49% | |||||||
| Adults | 18+ | 82-95% (across age groups) | 67-94% (across age groups) | 7-42% (across age groups) | 67-96% (across age groups) | 64-95% (across age groups) | 83-99%
(across age groups)
|
||
*In the last 6 months, primary series completed or booster dose received
Vaccine effectiveness
Between August 29, 2022, and September 25, 2022, unvaccinated Canadians, including children, were three times more likely to be hospitalized and five times more likely to die from their illness, compared to those who had two or three vaccine doses (20). This was true for children ages 5-17, in which COVID-19 cases were higher among the unvaccinated compared to those who had completed a primary vaccine series (20). Indeed, looking at the numbers, vaccines have proven effective: only 5.1% of children with a completed primary series had COVID-19, while 75% of unvaccinated children ages 5-11 had COVID-19. Among kids 5-11 who got additional boosters, there were no cases of COVID-19 reported by March 2023 (20). In the 12-17 age bracket, only 1.9% of those with a booster got COVID-19, whereas 52.9% of those unvaccinated had COVID-19. There were no cases observed in the 12- to 17-year-olds with two boosters (20). Further booster doses reduced the likelihood of being diagnosed with COVID-19 (20). One caveat to these numbers: many children and teens who got COVID-19 may not have reported a positive rapid antigen test (RAT) result to government authorities.
By comparison, in the United States in December 2021 at the very start of Omicron, the monthly hospitalization rate among unvaccinated adolescents aged 12-17 (23.5 per 100,000 adolescents) was six times that of fully vaccinated adolescents (3.8 per 100,000 adolescents) (21). This further demonstrates that vaccination protects youths against adverse outcomes.
Recent studies have also confirmed vaccine effectiveness against SARS-CoV-2 infection. Vaccine efficacy of three doses of the Pfizer vaccine in children 6 months to 4 years of age was 73.2% (22). In ages 5-11 years, two doses of COVID-19 vaccine was associated with lower risks of SARS-CoV-2 infections with or without symptoms, hospitalizations, and MIS-C (compared to unvaccinated children) (23). Another study showed similar findings in the 5-11 age group: the estimated vaccine effectiveness against all infections (including Omicron subvariants) after two doses of the Pfizer vaccine was 57.6%, 15 to 30 days after vaccination. The effectiveness against critical infection (intensive care unit admission or death) remained 100% after three months (24).
Factors affecting COVID-19 vaccine acceptance and uptake in children
CITF-funded studies have highlighted that vaccine uptake in the pediatric population is strongly influenced by parents’ beliefs and acceptance of COVID-19 vaccines. Those who were unlikely to vaccinate their children shared concern over the lack of information about the vaccine’s safety and potential side effects (71%) and the belief that their child would not get seriously ill from COVID-19 (36%) (25). Confidence in vaccination was associated with the child’s age (unpublished results). For example, 50% of parents of 2- to 3-year-olds intended to have their child vaccinated, whereas nearly 80% of parents of 8- to 9-year-olds were very likely to do so. Nearly 90% of the parents of 14- to 18-year-olds intended to have their adolescents vaccinated, if they had not already done so (26).
The EnCORE study reported that social determinants (education, household income, race/ethnicity, birthplace, and neighbourhood) were associated with children and adolescent vaccine acceptance (25):
- Children ages 5 to 11, whose parents identified as a racial or ethnic minority, who lived in homes with a higher number of people per bedroom, and who had a household income below $100,000, tended to have higher infection-acquired seroprevalence (8).
- Between November 2021 and January 2022, one of Montreal’s poorest and most racially diverse neighborhoods, Montreal North, reported the highest seroprevalence (16.7%) among children in the city (6, 27). Mercier-Hochelaga-Maisonneuve (HOMA), where nearly one-third of the population lives below the poverty line, reported 11.6% seroprevalence (6, 27). The West Island, with a more affluent and educated population reported 3.2% seroprevalence (6, 27). These differences were observed from the start of the study in October 2020 (28) and are similar to what was observed in adults.
- In households with less than $100,000 annual income, children had 18.4% lower prevalence of being vaccinated compared to households with more than $150,000 annually.
- Adolescents from the most deprived neighbourhoods were half as likely to be vaccinated as those from the least deprived neighbourhoods.
- Parents born outside Canada were more likely to report their child was unlikely to be vaccinated compared to Canadian-born parents.
- Racialized parents reported greater unwillingness to vaccinate their children than white parents.
Similarly, the TARGet Kids! cohort in Toronto revealed that lower family income and lack of university education were associated with 3-fold higher odds that parents believed COVID-19 vaccines are not safe and/or not important for children (unpublished results).
Canadian studies corresponded well with UK findings, where, as of July 2022 (29):
- 82% of school children who had at least one parent with three doses had at least one dose. Among school children whose parents were unvaccinated, only 5.3% had at least one dose.
- The vaccination rate was 44.8% among children living in the most deprived areas of England, compared with 80.7% in the least deprived areas.
- School children of Chinese and Indian descent were most likely to have received a dose (83.5% and 75.7%, respectively) while Gypsy/Roma and Black Caribbean children were less likely (15.8% and 16.5%, respectively), which is at least partly related to different levels of material deprivation (29).
By comparison, an American survey conducted in October 2021 among non-Hispanic white parents found that, whether or not the parents were vaccinated, their misconceptions about COVID-19, general vaccine mistrust, and attitudes toward collective responsibility and individual freedoms accounted for almost half of the variability in their children’s vaccine status (30).
Among the barriers affecting pediatric vaccination uptake in the US are concerns about side effects for young children, the perception that FDA approval was rushed, overall distrust of government and pharmaceutical companies, lack of community and family support for vaccinating children against COVID-19, and conflicting media messaging. Vaccine-resistant and hesitant parents were more likely to believe that children were not susceptible to infection and that the vaccines were ineffective against new variants (31).
The SPRING Study found that the key factors for increasing vaccine confidence were providing information on vaccine safety and benefits, leveraging trusted voices (such as provincial health officers), and encouraging individuals to promote vaccination among friends/social networks (26).
This aligns with other studies suggesting the benefits of improved government messaging and transparency, and increased health literacy surrounding vaccines. The idea that public health scientists and healthcare providers engage with communities to provide critical sources of vaccine information is recurrent. There is a need for pediatricians to communicate adequately and pay attention to parental concerns to better improve COVID-19 vaccine uptake for young children (31). Lastly, using real people’s stories about good vaccination experiences and disseminating them through social media is another mechanism to deliver a positive message about vaccination (32).
Vaccine side effects in children
The potential risks of COVID-19 illness far outweigh the side effects of COVID-19 vaccination. Side effects from COVID-19 vaccines in children and youth are similar to those for adults (33-35).
| Common vaccine side effects may include (35, 36): | |
| Symptoms at the injection site | More general symptoms |
|
|
- Babies aged 6 months to 3 years old also might cry, feel sleepy or lose their appetite after vaccination (34).
- Similar to adults, children usually have side effects within 2 days of vaccination that typically last 1 to 3 days.
- More children reported side effects, other than injection site pain, after the second dose. Reactions are often mild and resolve themselves within hours or days.
As with all vaccines, there’s a very low chance of a serious side effect. Although millions of children in Canada and around the world have been safely vaccinated, some rare reactions have been reported. As of March 3, 2023 (37):
- 114 adverse events were observed in children under 4 years old – out of 309,783 vaccine doses given to children in this age group (0.04%).
- 5- to 11-year-olds accounted for 794 adverse events out of 3,482,007 vaccine doses (0.02%).
- 1,725 adverse events were observed among those aged 12-17, out of 5,303,679 vaccine doses (0.03%).
The CITF-funded Canadian Immunization Monitoring Program ACTive (IMPACT), led by Drs. Karina Top, Shaun Morris, Fatima Kakkar, Julie Bettinger, Manish Sadarangani, and Scott Halperin is one of the organizations reporting serious adverse events following immunization (AEFI) among children to the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS). As of January 6, 2023, IMPACT reported 125 AEFIs to CAEFISS, of which 106 required hospitalization and were considered serious. IMPACT cases accounted for 35% of serious AEFI reports to CAEFISS among children.
The most common types of AEFIs were myocarditis (37%), myopericarditis (11%), MIS-C (11%), and pericarditis (6%); <5 cases of seizures were reported. No deaths were reported. 71% of cases were in males, with a median age of 17 years. 58% of cases occurred after dose 2, consistent with the groups with higher rates of myocarditis, myopericarditis, and pericarditis (unpublished results).
Myocarditis and pericarditis – a rare adverse effect after COVID-19 vaccination in kids
A CITF-funded study (38) in Ontario reported that the risk of myocarditis or pericarditis after receiving the Pfizer mRNA vaccine remains very rare (<0.01% in those aged 12-15 and <0.1 in those aged 16-17), with no deaths reported. Furthermore, the incidents of myocarditis/pericarditis were more frequent after the second dose than after the first dose. Studies from the US and UK corroborate the Canadian findings (33, 39, 40).
Multisystem inflammatory syndrome in children (MIS-C)
Some cases of MIS-C have been reported following vaccination, which led to hospitalization, but no deaths:
- As of January 6, 2023, the IMPACT study reported 12 cases of MIS-C out of a total of 9 million vaccine doses administered to children (unpublished results).
- In a study from the US (41), as of August 31, 2021, the reported rate for MIS-C in people under 18 who may have had COVID-19, but who also had one or more doses of vaccination, was 1 case per million. The reported rate among those under 18 who were vaccinated, but who never had COVID-19, was 0.3 cases per million.
- A separate study from the US recently published in Jama Network reported no serious adverse effects in patients with a prior diagnosis of MIS-C who were eligible for COVID-19 vaccination (age ≥5 years; ≥90 days after MIS-C diagnosis).
CITF-funded researchers foresee challenges facing children
Asked to consider the challenges in the near-term with respect to the pediatric population, CITF-funded experts raised the following concerns:
- Top, Morris, Kakkar, and Jonathan McGuire of the IMPACT Study mentioned that low vaccine coverage and high vaccine hesitancy will continue to affect immune protection among children. Dr. Hélène Decaluwe of the Immune Response in Young ImmunoSuppressed children to COVID-19 vaccination (IRYIS) Study echoed this concern and suggested the importance of educating parents about the potential dangers arising from complications from COVID-19 disease.
- IMPACT Study researchers raised the prospect that future variants could induce more severe symptoms to SARS-CoV-2, adding that this could emerge as a significant challenge in the near future. They also pointed out that the pandemic has exposed shortcomings in the healthcare system, including the need for greater in-patient pediatric capacity and investments to deal with the longer-term outcomes of COVID-19. Dr. Elinor Simons from the CHILD Cohort Study expressed a general concern with the lack of adequate nursing in the healthcare system.
- Decaluwe is also concerned about significant increases observed in the number of children and teens who have been diagnosed with autoimmune and neuroinflammatory diseases since the beginning of the pandemic, though it remains to be determined whether there is a causal relationship.
- It remains important to ascertain the reasons behind vaccine-associated myocarditis/pericarditis observed in the younger population, according to Dr. Decaluwe, despite the rarity of this issue. Consequently, there is a need to better understand whether vaccination in this population will imprint a restricted immune response to future coronaviruses, and how that could affect future immunity.
- Researchers from the CHILD Cohort Study, led by Dr. Megan Azad, have highlighted that recent lockdown restrictions led to reduced social interactions within daycares and schools – something that could delay a child’s social development. Additional focus on these socioemotional skills is required to facilitate catch up, said Dr. Leslie Roos (Clinical Psychologist, University of Manitoba).
- Additionally, Dr. Emily Cameron (University of Manitoba) who works with the CHILD Study as well, mentioned that rates of mental health issues increased in children and adolescents during the COVID-19 pandemic, with heightened vulnerability for youth with pre-pandemic emotional and behavioural concerns and those whose parents also experienced psychological distress. In many cases, psychological interventions will be needed to remediate these concerns; however, there continues to be barriers to widespread service access.
Concluding remarks
Omicron has changed the nature of the pandemic for children and adolescents. Whereas it could once be said that the pediatric population was unlikely to become seriously ill, the spread of the virus has now made it sufficiently dangerous that pediatric vaccination is essential.
Vaccine hesitancy among parents is of concern because it impedes these efforts. Vaccines have proven to be overwhelmingly safe. Any risk of adverse effects should be considered in relation to the benefits of vaccines and the risks posed by COVID-19 disease. That being said, children with underlying conditions and other special medical needs should only be vaccinated following consultation with a pediatrician.
References
- Government of Canada. COVID-19 epidemiology update: Current situation [Internet]. Ottawa, ON: Government of Canada; [updated 2023 Mar 13; cited 2023 Mar 17]. Available from: https://health-infobase.canada.ca/covid-19/current-situation.html.
- O’Brien SF, Caffrey N, Yi QL, Pambrun C, Drews SJ. SARS-CoV-2 seroprevalence among Canadian blood donors: The advance of omicron. Viruses. 2022;14(11).
- COVID-19 Immunity Task Force. The importance of pediatric vaccination [Internet]. [Available from: https://www.covid19immunitytaskforce.ca/wp-content/uploads/2022/03/CITF_CanCOVID_6_Pediatric-vaccination_2022_EN_FINAL-2.pdf.
- COVID-19 Immunity Task Force. COVID-19’s Youngest Victims [Available from: https://www.covid19immunitytaskforce.ca/wp-content/uploads/2023/03/citf-pediatrics-seminar-en.pdf.
- Skowronski DM, Kaweski SE, Irvine MA, Kim S, Chuang ESY, Sabaiduc S, et al. Serial cross-sectional estimation of vaccine-and infection-induced SARS-CoV-2 seroprevalence in British Columbia, Canada. CMAJ. 2022;194(47):E1599-e609.
- EnCORE Study. EnCORE Report November – December 2021 Round 3: Preliminary results [Internet]. Montreal, QC: EnCORE Study; [cited 2023 Mar 17]. Available from: https://www.encorestudy.ca/results].
- EnCORE Study. EnCORE Report May – September 2022 Round 4: Preliminary results [Internet]. Montreal, QC: EnCORE Study; [cited 2023 Mar 17]. Available from: https://www.encorestudy.ca/results.
- Zinszer K, Charland K, Pierce L, Saucier A, McKinnon B, Hamelin M-È, et al. Seroprevalence, seroconversion, and seroreversion of infection-induced SARS-CoV-2 antibodies among a cohort of children and adolescents in Montreal, Canada. medRxiv. 2022.
- Centers for Disease Control and Prevention. COVID Data Tracker: Rates of laboratory-confirmed COVID-19 hospitalizations by vaccination status [Internet] Atlanta, GA: US Department of Health and Human Services; [cited 2023 Mar 19]. Available from: https://covid.cdc.gov/covid-data-tracker/#covidnet-hospitalizations-vaccination.
- Zambrano LD, Newhams MM, Olson SM, Halasa NB, Price AM, Boom JA, et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12-18 Years – United States, July-December 2021. MMWR Morb Mortal Wkly Rep. 2022;71(2):52-8.
- Levy M, Recher M, Hubert H, Javouhey E, Fléchelles O, Leteurtre S, et al. Multisystem Inflammatory Syndrome in children by COVID-19 vaccination status of adolescents in France. JAMA. 2022;327(3):281-3.
- Block JP, Boehmer TK, Forrest CB, Carton TW, Lee GM, Ajani UA, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination – PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517-23.
- Patone M, Mei XW, Handunnetthi L, Dixon S, Zaccardi F, Shankar-Hari M, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28(2):410-22.
- Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, et al. Safety of the BNT162b2 mRNA covid-19 vaccine in a nationwide setting. New England Journal of Medicine. 2021;385(12):1078-90.
- Laverty M, Salvadori M, Squires SG, Ahmed M, Eisenbeis L, Lee S, et al. Multisystem inflammatory syndrome in children in Canada. Can Commun Dis Rep. 2021;47(11):461-5.
- Watanabe A, Iwagami M, Yasuhara J, Takagi H, Kuno T. Protective effect of COVID-19 vaccination against long COVID syndrome: A systematic review and meta-analysis. Vaccine. 2023;41(11):1783-90.
- Messiah SE, Hao T, DeSantis SM, Swartz MD, Talebi Y, Kohl HW, 3rd, et al. Comparison of persistent symptoms following SARS-CoV-2 infection by antibody status in nonhospitalized children and adolescents. Pediatr Infect Dis J. 2022;41(10):e409-e17.
- Centers for Disease Control and Prevention. COVID Data Tracker: Trends in demographic characteristics of people receiving COVID-19 vaccinations in the United States [Internet] Atlanta, GA: US Department of Health and Human Services; [updated 2023 Mar 15; cited 2023 Mar 17]. Available from: https://covid.cdc.gov/covid-data-tracker.
- Coronavirus (COVID-19) in the UK. Vaccinations in England [Internet]. [updated 2023 Mar 16; cited 2023 Mar 17]. Available from: https://coronavirus.data.gov.uk/details/vaccinations?areaType=nation&areaName=England.
- Government of Canada. COVID-19 epidemiology update: Cases following vaccination [Internet]. [updated 2023 Mar 13; cited 2023 Mar 17]. Available from: https://health-infobase.canada.ca/covid-19/cases-following-vaccination.html.
- Marks KJ, Whitaker M, Anglin O, Milucky J, Patel K, Pham H, et al. Hospitalizations of children and adolescents with laboratory-confirmed COVID-19 – COVID-NET, 14 States, July 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):271-8.
- Walter EB, Talaat KR, Sabharwal C, Gurtman A, Lockhart S, Paulsen GC, et al. Evaluation of the BNT162b2 covid-19 vaccine in children 5 to 11 Years of age. N Engl J Med. 2022;386(1):35-46.
- Watanabe A, Kani R, Iwagami M, Takagi H, Yasuhara J, Kuno T. Assessment of efficacy and safety of mRNA COVID-19 vaccines in children aged 5 to 11 years: A systematic review and meta-analysis. JAMA Pediatr. 2023.
- Jang EJ, Choe YJ, Kim RK, Park YJ. BNT162b2 vaccine effectiveness against the SARS-CoV-2 Omicron variant in children aged 5 to 11 years. JAMA Pediatr. 2023;177(3):319-20.
- McKinnon B, Quach C, Dubé È, Tuong Nguyen C, Zinszer K. Social inequalities in COVID-19 vaccine acceptance and uptake for children and adolescents in Montreal, Canada. Vaccine. 2021;39(49):7140-5.
- COVID-19 Immunity Task Force. Risks and impacts of the COVID-19 pandemic on Canada’s kids, their parents, and teachers: Latest research results and policy implications [Internet]. [Available from: https://www.covid19immunitytaskforce.ca/wp-content/uploads/2021/09/CITF_CanCOVID_Seminar1_FINAL_EN.pdf.
- Statistics Canada. Census profile, 2016 Census: Montréal [Census metropolitan area], Quebec and Canada [Country] [Internet]. [updated 2017 Nov 29; cited 2023 Mar 17]. Available from: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/page.cfm?Lang=E&Geo1=CMACA&Code1=462&Geo2=PR&Code2=01&Data=Count&SearchText=Montreal&SearchType=Begins&SearchPR=01&TABID=1&B1=All.
- Zinszer K, McKinnon B, Bourque N, Pierce L, Saucier A, Otis A, et al. Seroprevalence of SARS-CoV-2 antibodies among children in school and day care in Montreal, Canada. JAMA Network Open. 2021;4(11):e2135975-e.
- Office for National Statistics (ONS). Coronavirus (COVID-19) vaccination uptake in school pupils, England: up to 22 July 2022 [Internet]. [cited 2023 Mar 17]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandwellbeing/articles/coronaviruscovid19vaccinationuptakeinschoolpupilsengland/upto22july2022.
- Gray A, Fisher CB. Determinants of COVID-19 Vaccine uptake in adolescents 12-17 Years old: Examining pediatric vaccine hesitancy among racially diverse parents in the United States. Front Public Health. 2022;10:844310.
- Fisher CB, Bragard E, Jaber R, Gray A. COVID-19 vaccine hesitancy among parents of children under five years in the United States. Vaccines (Basel). 2022;10(8).
- Schilling S, Orr CJ, Delamater AM, Flower KB, Heerman WJ, Perrin EM, et al. COVID-19 vaccine hesitancy among low-income, racially and ethnically diverse US parents. Patient Educ Couns. 2022;105(8):2771-7.
- Government UK. Research and analysis Coronavirus vaccine – summary of Yellow Card reporting [Internet]. [updated 2023 Mar 8; cited 2023 Mar 17]. Available from: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting.
- Mayo Clinic. COVID-19 vaccines for kids: What you need to know [Internet]. [cited 2023 Mar 17]. Available from: https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/covid-19-vaccines-for-kids/art-20513332.
- Government of Canada. Vaccines for children: COVID-19 [Internet]. [updated 2023 Jan 20; cited 2023 Mar 17]. Available from: https://www.canada.ca/en/public-health/services/vaccination-children/covid-19.html?utm_campaign=hc-sc-covidvaccine-22-23&utm_medium=sem&utm_source=bing&utm_content=ad-text-en&utm_term=side%20effects%20of%20covid%20vaccine%20in%205%2011%20year%20olds&adv=2223-249950&id_campaign=396742155&id_source=1256742395035133&id_content=78546549749570&gclid=4a0ef62755031756d36992343b0986ec&gclsrc=3p.ds&.
- Canadian Immunization Research Network. CANVAS-COVID study results [Internet] [updated 2022 Sep 7; cited 2023 Mar 17]. Available from: https://canvas-covid.ca/results/pediatric-results/.
- Public Health Agency of Canada. Canadian COVID-19 vaccination safety report [Internet]. Ottawa, ON: Public Health Agency of Canada; [updated 2023 Mar 17; cited 2023 Mar 17]. Available from: https://health-infobase.canada.ca/covid-19/vaccine-safety/.
- Buchan SA, Alley S, Seo CY, Johnson C, Kwong JC, Nasreen S, et al. Myocarditis or pericarditis events after BNT162b2 vaccination in individuals aged 12 to 17 Years in Ontario, Canada. JAMA Pediatrics. 2023.
- National Center for Immunization and Respiratory Diseases (NCIRD). Selected adverse events reported after COVID-19 vaccination [Internet]. CDC; [updated 2023 Mar 7; cited 2023 Mar 17]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html.
- Office for National Statistics (ONS). COVID-19 vaccination and mortality in young people during the coronavirus pandemic [Internet]. [updated 2022 Mar 22; cited 2023 Mar 17]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/causesofdeath/articles/covid19vaccinationandmortalityinyoungpeopleduringthecoronaviruspandemic/2022-03-22].
- Yousaf AR, Cortese MM, Taylor AW, Broder KR, Oster ME, Wong JM, et al. Reported cases of multisystem inflammatory syndrome in children aged 12-20 years in the USA who received a COVID-19 vaccine, December, 2020, through August, 2021: a surveillance investigation. Lancet Child Adolesc Health. 2022;6(5):303-12.

