Colwill K, Galipeau Y, Stuible M, Gervais C, Arnold C, Rathod B, Abe KT, Wang JH, Pasculescu A, Maltseva M, Rocheleau L, Pelchat M, Fazel-Zarandi M, Iskilova M, Barrios-Rodiles M, Bennett L, Yau K, Cholette F, Mesa C, Li AX, Paterson A, Hladunewich MA, Goodwin PJ, Wrana JL, Drews SJ, Mubareka S, McGeer AJ, Kim J, Langlois M-A, Gingras A-C, Durocher Y. A scalable serology solution for profiling humoral immune responses to SARS-CoV-2 infection and vaccination. Clin Transl Immunol 2022 Mar 23. doi: 10.1002/cti2.1380.
The results and/or conclusions contained in the research do not necessarily reflect the views of all CITF members.
In a paper now published in Clinical and Translational Immunology, a team of Canadian researchers partly funded by the CITF and led by Drs. Anne-Claude Gingras from Toronto’s Lunenfeld-Tanenbaum Research Institute, Marc-André Langlois from the University of Ottawa, and Yves Durocher from the National Research Council of Canada, has developed a set of standardized, high-throughput serological assays to detect antibodies against SARS-CoV-2. Multiple serosurveys underway in Canada – including over half of those funded by the CITF – use this platform, making it possible to harmonize and compare their results nationwide. The authors describe their multi-faceted detection of the antibody response to SARS-CoV-2 infection and vaccination.
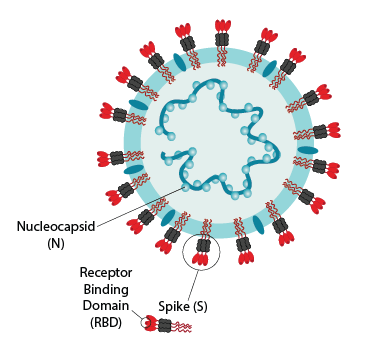
Figure 1. A SARS-CoV-2 viral particle with labelled nucleocapsid (N) and spike (S) proteins. The virus’ receptor binding domain (RBD), housed on the S protein, is also shown. The tests developed by Drs. Gingras and Langlois can detect antibodies against all three of these viral antigens.
This unique made-in-Canada platform enables the detection of IgG antibodies recognizing the SARS-CoV-2 spike (S) trimer, its receptor binding domain (RBD), and SARS-CoV-2 nucleocapsid (N) proteins (see figure below) as well as detection of antibodies that neutralize the virus. It has profiled over 150,000 unique samples and well over half of CITF-funded serosurveys use this platform. Most importantly, the harmonized high-throughput serological assays enable the comparison of data and aggregation of results nationwide.
Key points:
- Protocols developed can be used to detect, in high-throughputHigh-throughput describes the scaling-up and automation of laboratory tests to process large numbers of samples at once., antibodies to the main SARS-CoV-2 antigens (S, RBD, and N) from serum and dried blood spots (DBS). A surrogate virus neutralization assay detects antibodies capable of disrupting the interaction between S and the ACE2 receptor.
- All reagents used were generated at the National Research Council of Canada, and are made available across the country to enable harmonization of standardized, automated high-throughput or manual serological assays. This has the advantage of enabling researchers to compare and aggregate data.
- The automated ELISAsAn enzyme-linked immunosorbent assay (ELISA) is a laboratory technique used to detect the presence of antigens in a biological sample. An ELISA, like other types of immunoassays, relies on antibodies to detect a target antigen (e.g., viral proteins) using highly specific antibody-antigen interactions. have been optimized in two independent sites: Toronto (Gingras lab) and Ottawa (Langlois lab).
- hese assays have been calibrated using reference standards from the World Health Organization to provide quantitative outputs in binding antibody units (BAU). This enables cross-comparison of serological responses worldwide to better evaluate vaccination strategies and the evolution of global immunity to SARS-CoV-2

