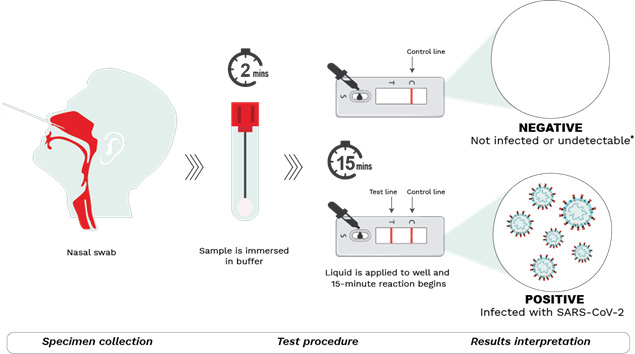
Your COVID-19 questions answered
We assembled our experts to offer responses to some of the most pressing questions regarding the current state of the pandemic. From the startling emergence of the Omicron variant to the use of rapid antigen tests to the recommendation and timing of third dose booster vaccines. The evidence collected by CITF-funded research so far has helped to inform health care and public health decisions with regard to the ever-changing situation. We hope that it will help you to make informed choices regarding your health and to stay safe.
References
- Pegu A, O’Connell SE, Schmidt SD, O’Dell S, Talana CA, Lai L, et al. Durability of mRNA-1273 vaccine–induced antibodies against SARS-CoV-2 variants. Science. 2021;373(6561):1372-7.
- Naaber P, Tserel L, Kangro K, Sepp E, Jürjenson V, Adamson A, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10:100208.
- Zhang A, Breznik JA, Clare R, Nazy I, Miller MS, Bowdish DME, et al. Antibody Responses to Third-Dose mRNA Vaccines in Nursing Home and Assisted Living Residents. J Am Med Dir Assoc. 2022.
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465-9.
- Bekliz M, Perez-Rodriguez F, Puhach O, Adea K, Melancia SM, Baggio S, et al. Sensitivity of SARS-CoV-2 antigen-detecting rapid tests for Omicron variant. medRxiv. 2022:2021.12.18.21268018.
- Sheikh A KS, Woolhouse M, McMenamin J, Robertson C. Severity of Omicron variant of concern and vaccine effectiveness against symptomatic disease: national cohort with nested test negative design study in Scotland. 2021 Dec 22.
- Wolter N, Jassat W, Walaza S, Welch R, Moultrie H, Groome M, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022.
- Abu-Raddad LJ, Chemaitelly H, Bertollini R. Severity of SARS-CoV-2 Reinfections as Compared with Primary Infections. N Engl J Med. 2021;385(26):2487-9.