By Mariana Bego
Two London-based studies have shown that people who have been infected and developed an immune response to SARS-CoV-2 react briskly to a first dose of vaccine, similar to how uninfected (i.e., infection naïve) individuals would respond to a second (booster) dose (Figure 1). The results of both these studies were published in The Lancet at the end of February. They join two pre-prints released earlier last month from preliminary studies performed in the United States.
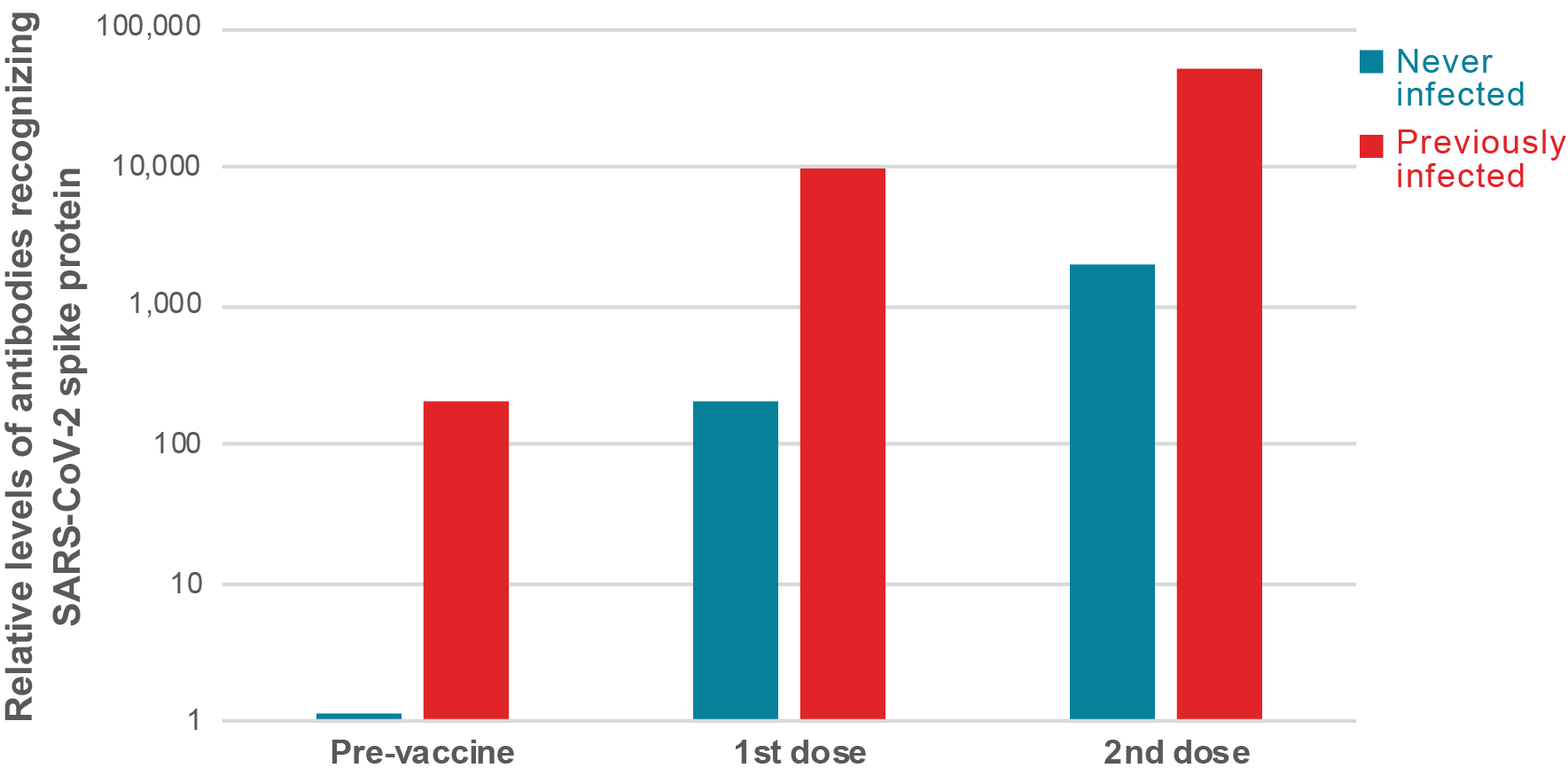
Figure 1: People previously infected with SARS-CoV-2 virus mount a strong immune response after just one dose of vaccine. On average, the different studies show that people with previous exposure to SARS-CoV-2 have similar levels of antibodies prior to vaccination than naïve people after one dose, while after the first dose, their antibody levels are between 25 and 100 times higher. One publication showed that after the second dose, people previously infected with SARS-CoV-2 have over 10 times more antibodies compared to naïve individuals.
Individuals that recover from SARS-CoV-2 infection make significantly more antibodies in response to a single dose of vaccine than naïve individuals
Investigators from University College London and Public Health England reasoned that previous infection could be analogous to immune priming. They analyzed 51 individuals from a longitudinal cohort study of health care workers in London who underwent weekly PCR tests and quantitative serology testing every 16 weeks beginning in March 2020. Almost half of these (24 individuals) had a previous laboratory-confirmed mild or asymptomatic SARS-CoV-2 infection. All of the subjects were tested for antibodies 19 to 29 days after receiving their first dose of the Pfizer-BioNTech vaccine. The levels of antibodies recorded in naïve individuals after one vaccine dose were comparable to the peak of antibodies detected in individuals with a previous natural infection who had not yet been vaccinated (in average approximately 100 arbitrary units [AU]/ml for both). After vaccination, those who had a previous natural infection increased their antibody levels to more than 140 times (in average 14,000 AU/ml).
The authors of this study recommend that, to potentially accelerate vaccine rollout, priority should be given to individuals with no previous infection. They go on to suggest that this could be facilitated and implemented by including serology testing at the time of first vaccination. The objective would be to achieve faster and wider coverage without compromising vaccine-induced immunity in order to reduce variant emergence.
Read their correspondence here.
Research conducted by investigators at the Icahn School of Medicine at Mount Sinai New York described the antibody responses in over 100 individuals, 41 of whom had documented pre-existing SARS-CoV-2 immunity and 68 who were infection naïve. All individuals received their first dose of either the Moderna or Pfizer-BioNTech mRNA vaccine at the end of 2020. They observed that the antibody levels of vaccinees with pre-existing immunity were not only approximately 100 times higher than those of naïve vaccinees, but also exceeded the median antibody levels measured in naïve individuals after the second vaccine dose by almost 10 fold (see figure 1). Of note, and in agreement with the massive immune response recorded, vaccine recipients with pre-existing immunity experienced more severe systemic vaccination side effects than antibody naïve vaccine recipients. Future follow-up studies will need to address whether these early differences in immune responses are maintained over time.
Read their pre-print here.
Researchers in both studies suggest that policies tailored to assess previous immune response to SARS-CoV-2 would not only allow for the expansion of the limited vaccine supply, but would also limit the vaccine side effects experienced by those recovered from COVID-19 and reduce vaccine hesitancy.
Similar results were echoed in a study done by researchers at the University of Maryland School of Medicine, published in JAMA this month. Their study followed 59 health care workers from date of vaccination (with either Moderna or Pfizer-BioNTech mRNA vaccines) to up to 2 weeks post-vaccination. As seen in other reports and following the same trends, people with prior COVID-19 infection showed statistically significant higher antibody levels compared to naïve individuals.
Read their manuscript here.
mRNA vaccines can elicit strong cellular response in people who recovered from COVID-19
A more in-depth study led by researchers at Imperial College London, followed 72 health care workers vaccinated in late December. All participants provided blood samples at the time of receiving their first dose of Pfizer-BioNTech’s coronavirus vaccine and 21 to 25 days after vaccination. Of these, 21 had evidence of prior coronavirus infection based on the presence of baseline serology (in 16 participants) or an early post-vaccine cellular immune response (in five participants). The remaining 51 participants, with no baseline serology and no early cellular responses post-vaccine, were defined as infection naïve.
As the vaccine they received encodes only for the spike glycoprotein of SARS-CoV-2, the researchers assessed immune responses to this protein post-vaccination. Antibodies levels recognizing the spike protein were on average 25 times higher in individuals with previous natural infection than in naïve individuals (on average over 16,000 AU/mL vs 600 AU/mL). Interestingly, the five participants who had early cellular responses but no serology at baseline, had intermediate levels of antibodies between the infection-naïve and previously infected groups. Similarly, vaccination-induced detectable neutralizing antibodies were detected in naïve individuals, but titers were significantly lower than for previously infected individuals. The 21 participants with evidence of previous SARS-CoV-2 infection mounted much stronger T-cell responses to spike peptides post-vaccination than naïve individuals. Furthermore, of the naïve group participants, only half had detectable T-cell responses. In summary, people with previous coronavirus infection appear to generate stronger immune responses, including cellular immune responses, to one dose of mRNA vaccine, compared to those who had not been previously infected.
In this cohort, some naïve individuals mounted a weak response to a single-dose vaccination. This was particularly the case for individuals older than 50 years of age. One naïve individual over the age of 50 and with low antibody titers post-vaccination (<65 AU/ml), developed COVID-19 a little over a month after the first dose of vaccine. The authors suggested that low antibody titers (under 250 AU/mL) may not provide sufficient immunity to protect from clinical disease or prevent the virus from spreading. They may not even persist until the second dose is administered.
The authors of this study recommended the prioritization of the second dose of vaccine for infection-naïve individuals over the age of 50, who are at an increased risk of both severe COVID-19 and may have lower protection from just one vaccine dose. Finally, their results also emphasize the need for continued rigorous use of personal protective equipment after vaccination to prevent both infection and asymptomatic spread of disease.
Read their article here.

